Hydrolysis of mixed Ni2+–Fe3+ and Mg2+–Fe3+ solutions and mechanism of formation of layered double hydroxides†
Abstract
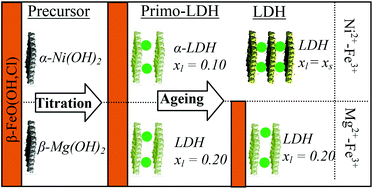
The hydrolytic behavior of mixed metallic solutions containing Ni2+–Fe3+ and Mg2+–Fe3+ has been studied with respect to the relative proportion of the divalent and trivalent cations in solution as well as the quantity of NaOH added. The combination of X-ray diffraction and vibrational spectroscopy provides a deep insight into both the nature of the phases and the structure of the formed LDH. The relative abundance of each phase is determined by using a mass balance diagram and is in good agreement with the solid characterization. We showed that the slow hydrolysis of mixed metallic solutions involved first the precipitation of Fe3+ to form an akaganeite phase, and then the formation of a precursor on the iron oxyhydroxide surface, which transforms into LDH by diffusion of FeIII species from the akaganeite phase to the precursor. Interestingly, whatever the iron content in solution, the same fraction of FeIII is incorporated into the LDH phase which is correlated to the nature of the formed precursor. For Ni2+–Fe3+ solution, the precursor is an α-Ni hydroxide, which formed a LDH phase with a very low iron content (xlayer = 0.1), but a high charge density provided by structural hydroxyl default. This result unambiguously demonstrated that the LDH phase is formed from the precursor structure. For Mg2+–Fe3+ solution, the precursor is structurally equivalent to a β-Mg(OH)2 phase, leading to a LDH with a higher xlayer value of ∼0.2. In both cases, at the end of the titration experiments, a mixture of different phases was systematically observed. Hydrothermal treatment allows the recovery of a pure LDH phase exclusively for the Ni2+–Fe3+ solution.

 Please wait while we load your content...
Please wait while we load your content...