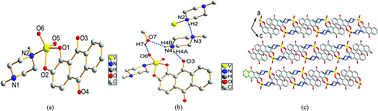
[VVO2(O2C14H6O2)(C5N2H12)](C5N2H13)(CH3OH) (1) and {Na[VVO(O2C14H6O2)2][(CH3)2NCHO]}n (2) have been synthesized by the reaction of V2O5 and NaVO3 with aromatic 1,2-diol (1,2-dihydroxyanthraquinone), and their molecular and crystal structures have been determined by X-ray diffraction. MTT assay tests of the VVO2LALB and VVOL2 complexes against cancer cells have revealed that, when L is catechol, VOL2 showed broad-spectrum, high anticancer activities which were proportional to their concentration; however when L is naphthol or alizarin, VOL2 displayed little effect towards the cancer cells; moreover, complex 1 in the coordination model of VVO2LALB showed specifically higher inhibition (88.65%) against HCT-8 than the clinical anticancer drug Fu-5 (69.97%). The results revealed that both the V(V) and the ligands cannot influence the inhibition against cancer cells individually. The mechanism of the broad-spectrum anticancer activities of VOL2 when L is catechol ligand might originate from the redox activities of VV/VIV which regulate the concentration of ROS (reactive oxygen species). N-Methylpiperazine formed as a by-product in complex 1 was confirmed by 1H NMR and its formation mechanism catalyzed by V2O5 has been deduced.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...