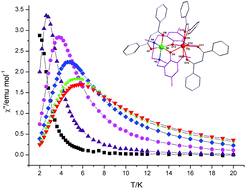
The synthesis and characterization of three isomorphous complexes [NiII(L)LnIII(DBM)3] (Ln = Gd (1·2.5CH3CN·0.5H2O), Tb (2·2CH3CN·0.5MeOH), and Dy (3·2CH3CN·0.5MeOH·0.5H2O)) are reported (H2L = N,N′-dimethyl-N,N′-(2-hydroxy-3-methoxy-5-methylbenzyl)ethylenediamine, DBM− = anion of 1,3-diphenylpropane-1,3-dione). The flexible ligand L2− contains an N2O2-inner and an O4-outer coordination site. There are diphenoxo bridges between NiII and LnIII ions in these complexes. The remaining coordination sites are occupied by DBM− anions. Direct current (dc) magnetic susceptibility measurements indicate the presence of intramolecular ferromagnetic interactions in desolvated complexes 1–3. The magnetic coupling constant JNiGd in complex 1 is estimated to be 1.11 cm−1 (H = −2JNiGdSNiSGd). Alternating current (ac) magnetic susceptibility studies reveal that complexes 2 and 3 show frequency-dependent out-of-phase signals, which indicate that they exhibit SMM behavior. The energy barriers for complexes 2 and 3 under a 2 kOe applied direct current (dc) magnetic field are estimated from Arrhenius plots to be 14.4 and 11.3 K, respectively.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...