Abstract
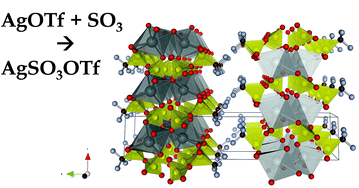
We describe the synthetic route towards a novel class of salts, trifluoromethylsulfonylsulfates, as exemplified by the silver(I) derivative (AgS2O6CF3). Formation proceeds via direct reaction between a triflate precursor, AgSO3CF3, and SO3. The title compound crystallizes in the P21/c unit cell with a = 5.15746(14) Å, b = 25.8563(9) Å, c = 5.53970(14) Å and β = 101.1749(19)°. The structure is layered with the puckered [AgS2O6] 2D sheets; the terminal CF3 groups are separated by the van der Waals gap, as seen also for related metal triflates. The compound is very fragile thermally and it decomposes endothermally to AgSO3CF3 with concomitant evolution of SO3 even at 65 °C or upon

 Please wait while we load your content...
Please wait while we load your content...