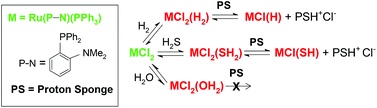
Thermodynamic data for the reversible formation of cis-RuCl2(P–N)(PPh3)(η2-H2) (2a) from trans-RuCl2(P–N)(PPh3) in C6D6 are determined by variable temperature 31P{1H} and 1H NMR spectroscopy; P–N = o-diphenylphosphino-N,N′-dimethylaniline. Values of ΔH° = −26 ± 4 kJ mol−1, ΔS° = −40 ± 15 J mol−1 K−1, and ΔG° (at 25 °C) = −13.8 ± 0.2 kJ mol−1 are compared with recently reported data for the corresponding H2S adduct (4a), where the exothermicity is greater by ∼20 kJ mol−1, but this is counteracted by a more unfavourable entropy change, and overall the K and ΔG° values at 25 °C are close. For loss of H2 from 2a in the solid state, whose X-ray structure is presented, ΔH° is 50 ± 3 kJ mol−1 as measured by Differential Scanning Calorimetry. The pKa values of the coordinated H2 (∼11) and H2S (∼14) are estimated by reactions of 2a and 4a with proton sponge (1,8-bis(dimethylamino)naphthalene) in CD2Cl2 at 20 °C; the mono-hydrido and -mercapto products are identified in situ. A corresponding H2O adduct is not deprotonated under the same conditions. Related dihydrido, mercapto and hydroxy species are formed by in situ reactions of 1a with NaH, NaSH, and NaOH, respectively.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...