Reactivity of C,N-chelated organoboron compounds with lithium anilides – formation of unexpected 1,2,3-trisubstituted 1H-2,1-benzazaboroles†
Abstract
A set of C,N-intramolecularly coordinated ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NtBu)C6H4], L2 = [o-(CH
NtBu)C6H4], L2 = [o-(CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-2,6-iPr2C6H3)C6H4], L3 = [o-(CH2NMe2)C6H4]); L1–3BCl2 (for 1 L = L1, for 2 L = L2, for 5 L = L3), L1BPhCl (3) and L1BCy2 (4) (where Cy =
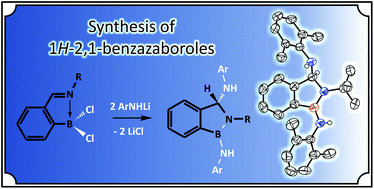
N-2,6-iPr2C6H3)C6H4], L3 = [o-(CH2NMe2)C6H4]); L1–3BCl2 (for 1 L = L1, for 2 L = L2, for 5 L = L3), L1BPhCl (3) and L1BCy2 (4) (where Cy = ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond yielding 1,2,3-trisubstituted 1H-2,1-benzazaboroles 6–11, whose structures were unambiguously established by
N bond yielding 1,2,3-trisubstituted 1H-2,1-benzazaboroles 6–11, whose structures were unambiguously established by ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond involved in the ligand backbones is the crucial step of the whole reaction. The C
N double bond involved in the ligand backbones is the crucial step of the whole reaction. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond in 1–3 is activated by its coordination to the ortho bonded Lewis acidic boron center, which was also proven by the fact that the non-substituted
N double bond in 1–3 is activated by its coordination to the ortho bonded Lewis acidic boron center, which was also proven by the fact that the non-substituted

 Please wait while we load your content...
Please wait while we load your content...