Origins of enantioselectivity in asymmetric ketone hydrogenation catalyzed by a RuH2(binap)(cydn) complex: insights from a computational study†
Abstract
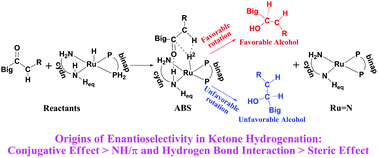
In this paper, the origins of enantioselectivity in asymmetric ketone hydrogenation catalyzed by RuH2(binap)(cydn) (cydn = trans-1,2-diaminocyclohexane) were discussed. Fifteen substrates involving aromatic, heteroaromatic, olefinic and dialkyl prochiral ketones were used to probe the catalytic mechanism and find an effective way to predict the chirality of the products. The calculated results demonstrate that the hydrogen transfer (HT) step from the Ru complex to the ketone substrate is the chirality-determining step in the H2-hydrogenation of ketones. The hydrogenation of aromatic-alkyl ketones can give higher enantiomeric excess (ee) values than that of dialkyl ketones. An interesting intermediate (denoted as ABS) could be formed if there is an α-hydrogen for R/R′ groups of the ketone due to the H2–Hα interaction. Two substituent groups of the ketone could rotate around the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O axis in two directions, clockwise or counter-clockwise. This rotation, with the big or conjugative substituent group away from/toward the closer binap ligand of the Ru catalyst, will form favorable/unfavorable chiral products with an Re-/Si- intermediate structure. On the contrary, if there is no such α-hydrogen in any substituent group of the ketone, ABS and another intermediate (denoted as INT) would not exist. This study indicates that the conjugative effect of the substituent groups of the ketone play an important role in differentiating the R/R′ groups of the ketone, while steric and electrostatic effects contribute to a minor extent. Furthermore, the disparity of the R and R′ groups of the ketone is of importance in the enantioselectivity and the favorable chiral alcohol is formed when the structure of the conjugative/big substituent group is away from the closer binap ligand of the RuH2(binap)(cydn) catalyst. According to the three factors of the substituent group and the fourth quadrant theory, the enantioselectivity of 91 prochiral ketones catalyzed by a series of Ru catalysts were predicted. All of the predictions are consistent with the experimental results.
O axis in two directions, clockwise or counter-clockwise. This rotation, with the big or conjugative substituent group away from/toward the closer binap ligand of the Ru catalyst, will form favorable/unfavorable chiral products with an Re-/Si- intermediate structure. On the contrary, if there is no such α-hydrogen in any substituent group of the ketone, ABS and another intermediate (denoted as INT) would not exist. This study indicates that the conjugative effect of the substituent groups of the ketone play an important role in differentiating the R/R′ groups of the ketone, while steric and electrostatic effects contribute to a minor extent. Furthermore, the disparity of the R and R′ groups of the ketone is of importance in the enantioselectivity and the favorable chiral alcohol is formed when the structure of the conjugative/big substituent group is away from the closer binap ligand of the RuH2(binap)(cydn) catalyst. According to the three factors of the substituent group and the fourth quadrant theory, the enantioselectivity of 91 prochiral ketones catalyzed by a series of Ru catalysts were predicted. All of the predictions are consistent with the experimental results.

 Please wait while we load your content...
Please wait while we load your content...