The new nickel tellurite chloride compound Ni15Te12O34Cl10 – synthesis, crystal structure and magnetic properties†
Abstract
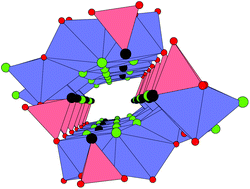
A new nickel tellurite oxohalide, Ni15Te12O34Cl10, has been prepared by chemical vapour transport reactions and the crystal structure was determined by ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with the pseudomonoclinic cell parameters a = 10.3248(6) Å, b = 10.3249(6) Å, c = 11.6460(8) Å, α = 73.782(6)°, β = 73.782(6)°, γ = 63.51(2)°, Z = 1, R1 = 0.0264. The Ni2+ ions have octahedral [NiO6] and [NiO4Cl2] coordinations, the Te4+ ions have one-sided [TeO3] and [TeO4] coordinations. The crystal structure can be described as consisting of nickel oxide ribbons extending along (001) that are connected by corner sharing [TeO3] and [TeO4] groups to build the open framework structure. The chlorine atoms and the Te-lone pairs are facing voids in the
with the pseudomonoclinic cell parameters a = 10.3248(6) Å, b = 10.3249(6) Å, c = 11.6460(8) Å, α = 73.782(6)°, β = 73.782(6)°, γ = 63.51(2)°, Z = 1, R1 = 0.0264. The Ni2+ ions have octahedral [NiO6] and [NiO4Cl2] coordinations, the Te4+ ions have one-sided [TeO3] and [TeO4] coordinations. The crystal structure can be described as consisting of nickel oxide ribbons extending along (001) that are connected by corner sharing [TeO3] and [TeO4] groups to build the open framework structure. The chlorine atoms and the Te-lone pairs are facing voids in the

 Please wait while we load your content...
Please wait while we load your content...