Group transfer reactions of d0 transition metal complexes: redox-active ligands provide a mechanism for expanded reactivity
Abstract
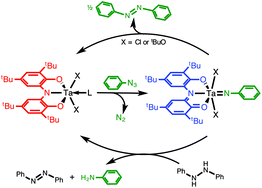
Group- and atom-transfer is an attractive reaction class for the preparation of value-added organic substrates. Despite a wide variety of known early-transition metal oxo and imido complexes, these species have received limited attention for atom- and group-transfer reactions, owing to the lack of an accessible metal-based two-electron redox couple. Recently it has been shown that redox-active ligands can support the multi-electron changes required to promote group-transfer reactivity, opening up new avenues for group- and atom-transfer catalyst design. This Perspective article provides an overview of group transfer reactivity in early-transition metal complexes supported by traditional ligand platforms, followed by recent advances in the atom- and group-transfer reactivity of d0 metal complexes containing redox-active ligands.

 Please wait while we load your content...
Please wait while we load your content...