PS2Br and AsS2X (X = Br, I): similar molecules with completely different shapes – a combined experimental and theoretical study†
Abstract
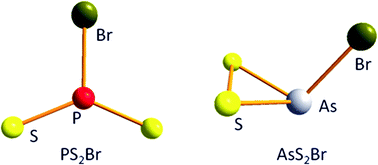
By the reaction of solid As4S4 with gaseous Br2 at a temperature of 410 K gaseous AsSBr and AsS2Br are formed; the reaction with gaseous I2 at 468 K leads to the formation of AsSI, AsS2I, As2S2I2 and As2S3I2. Thermodynamic data on these novel species are obtained by ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S π-bonds are realized due to their strength over one S–S σ-bond. As2S3I2 contains a five-membered As2S3-ring with one S–S-bond. The ionization energies are determined and compared with those obtained from calculations; they serve as a further indication for the structures realized in these molecules.
S π-bonds are realized due to their strength over one S–S σ-bond. As2S3I2 contains a five-membered As2S3-ring with one S–S-bond. The ionization energies are determined and compared with those obtained from calculations; they serve as a further indication for the structures realized in these molecules.

 Please wait while we load your content...
Please wait while we load your content...