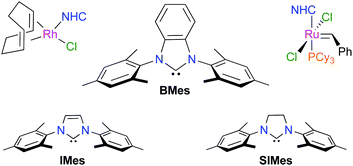
The deprotonation of 1,3-dimesitylbenzimidazolium tetrafluoroborate with a strong base afforded 1,3-dimesitylbenzimidazol-2-ylidene (BMes), which was further reacted in situ with rhodium or ruthenium complexes to afford three new organometallic products. The compounds [RhCl(COD)(BMes)] (COD is 1,5-cyclooctadiene) and cis-[RhCl(CO)2(BMes)] were used to probe the steric and electronic parameters of BMes. Comparison of the percentage of buried volume (%VBur) and of the Tolman electronic parameter (TEP) of BMes with those determined previously for 1,3-dimesitylimidazol-2-ylidene (IMes) and 1,3-dimesitylimidazolin-2-ylidene (SIMes) revealed that the three N-heterocyclic carbenes (NHCs) had very similar profiles. Nonetheless, changes in the hydrocarbon backbone subtly affected the stereoelectronic properties of these ligands. Accordingly, the corresponding [RuCl2(PCy3)(NHC)(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)] complexes displayed different catalytic behaviors in the ring-closing metathesis (RCM) of α,ω-dienes. In the benchmark cyclization of diethyl 2,2-diallylmalonate, the new [RuCl2(PCy3)(BMes)(
CHPh)] complexes displayed different catalytic behaviors in the ring-closing metathesis (RCM) of α,ω-dienes. In the benchmark cyclization of diethyl 2,2-diallylmalonate, the new [RuCl2(PCy3)(BMes)(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)] compound (1d) performed slightly better than the Grubbs second-generation catalyst (1a), which was in turn significantly more active than the related [RuCl2(PCy3)(IMes)(
CHPh)] compound (1d) performed slightly better than the Grubbs second-generation catalyst (1a), which was in turn significantly more active than the related [RuCl2(PCy3)(IMes)(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)] initiator (1b). For the formation of a model trisubstituted cycloolefin, complex 1d ranked in-between catalyst precursors 1a and 1b, whereas in the RCM of tetrasubstituted cycloalkenes it lost its catalytic efficiency much more rapidly.
CHPh)] initiator (1b). For the formation of a model trisubstituted cycloolefin, complex 1d ranked in-between catalyst precursors 1a and 1b, whereas in the RCM of tetrasubstituted cycloalkenes it lost its catalytic efficiency much more rapidly.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)] complexes displayed different catalytic behaviors in the ring-closing metathesis (
CHPh)] complexes displayed different catalytic behaviors in the ring-closing metathesis (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)] compound (1d) performed slightly better than the Grubbs second-generation
CHPh)] compound (1d) performed slightly better than the Grubbs second-generation ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh)] initiator (1b). For the formation of a model trisubstituted cycloolefin, complex 1d ranked in-between
CHPh)] initiator (1b). For the formation of a model trisubstituted cycloolefin, complex 1d ranked in-between 

 Please wait while we load your content...
Please wait while we load your content...