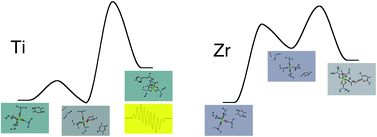
A series of group 4 metal tetracarbamates M(O2CNR2)4 (M = Ti, R = Et, 1a; M = Zr, R = Et, 1b; iPr, 1c; M = Hf, R = Et, 1d; R = iPr, 1e) were studied as catalytic precursors in the solution polymerization of rac-lactide. The titanium complex but not the zirconium and hafnium ones increase the activity by addition of iPrOH. The structure of the carbamato ligand markedly influences the molar mass of polymer; the complexes with isopropyl carbamato ligands produced PLA with molar masses up to 94 000 g mol−1. The main mechanistic aspects of the initial stages of the polymerization reactions were outlined by spectroscopic and computational analyses. In the case of zirconium and hafnium complexes, an interaction between a carbamato ligand and the CH unit of one lactide molecule is established at room temperature. This interaction is followed by the high temperature proton transfer from the lactide to the carbamato O-atom, affording a catalytic active alkoxy complex with release of CO2 and NHR2. The polymerization mediated by Ti(O2CNEt2)4 involves the release of a radical fragment [O2CNEt2]˙, with consequent generation of a Ti(III) center. The propagating chain is an alcoholate ligand coordinated to a Ti(IV) centre and containing a radical mainly localized at the tail of the chain (DFT, EPR).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...