Nickel nanoparticles catalyse reversible hydration of carbon dioxide for mineralization carbon capture and storage
Abstract
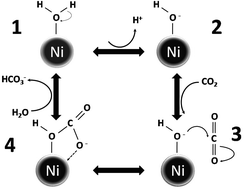
The separation and storage of CO2 in geological form as mineral carbonates has been seen as a viable method to reduce the concentration of CO2 from the atmosphere. Mineralization of CO2 to mineral salts like calcium carbonate provides a stable storage of CO2. Reversible hydration of CO2 to carbonic acid is the rate limiting step in the mineralization process. We report catalysis of the reversible hydration of CO2 using nickel nanoparticles (NiNPs) at room temperature and atmospheric pressure. The catalytic activity of the NiNPs is pH independent and as they are water insoluble and magnetic they can be magnetically separated for reuse. The reaction steps were characterized using X-ray photoemission spectroscopy and a possible reaction mechanism is described.

 Please wait while we load your content...
Please wait while we load your content...