Steam reforming of light oxygenates
Abstract
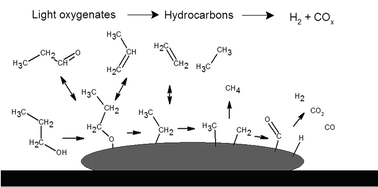
Steam reforming (SR) of ethanol, acetic acid, acetone, acetol, 1-propanol, and propanal has been investigated over Ni/MgAl2O4 at temperatures between 400 and 700 °C and at a steam-to-carbon-ratio (S/C) of 6. The yield of H2 and conversion increased with temperature, while the yield of by-products decreased with temperature in the SR of the investigated compounds. The yield of H2 approached the thermodynamic limit at the highest temperatures investigated. No significant differences in conversion as a function of temperature among the different model compounds were observed. However, the product distribution depended on the model compound, and C3-oxygenates produced a larger fraction of by-products compared to C2-oxygenates. Temperatures of 600 °C or above were generally needed to minimize the fraction of by-products and obtain a syngas containing mainly CO, CO2, H2, and H2O with only traces of CH4. Significant deactivation of the catalyst was observed for all of the compounds and was mainly due to carbon formation. The carbon formation was highest for alcohols due to a high formation of olefins, which are potent coke precursors.

 Please wait while we load your content...
Please wait while we load your content...