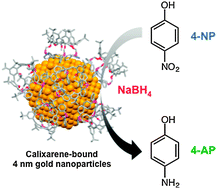
The homogeneous versus heterogeneous nature of the active site of gold catalysis of the 4-nitrophenol reduction to 4-aminophenol is investigated using poisoning experiments that employ various organic ligands and 4 nm gold nanoparticles as catalysts. DDT (dodecanethiol)-bound gold nanoparticles are unable to catalyze this reaction, whereas nanoparticles capped with calixarene ligands consisting of calix[6]arene phosphine C6P and calix[4]arene thiol MBC are active. Poisoning of residual terrace sites upon addition of 2-naphthalenethiol (2-NT) in these latter two catalysts results in a gold nanoparticle that consists solely of ostensibly similar pinhole defect sites. However, the reaction rate for the catalyst consisting of C6P + 2-NT is 6.5-fold higher relative to the rate for catalyst consisting of MBC + 2-NT. This observation along with lack of activity for the DDT-bound catalyst suggests that pinhole defect sites and the gold nanoparticle surface cannot be the active site for catalysis. Instead, the active site is suggested to be a leached gold species that is present in exceedingly small concentrations (cannot be detected by disappearance of gold nanoparticles from solution during catalysis). Such a supposition is supported by observations of induction time and the interplay between observed induction time and kinetics. It is observed that the composition of the organic ligands in the system controls the kinetics and the induction time. Additionally, there is an absence of an induction time in a solution containing used catalyst, to which reactants are added. In the initial catalysis, it is observed that as the thiol ligand concentration on the surface increases, the induction time increases and the reaction rate decreases. The leached species were unable to be detected via changes in the surface-plasmon resonance absorption of the gold nanoparticles in solution before and after catalysis, as well as electron microscopy studies of used nanoparticle catalysts. This suggests that the leached species concentration is low, and their catalytic activity in turn must be quite high.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...