Combustion synthesis of LaMn1−xAlxO3+δ (0 ≤ x ≤ 1): tuning catalytic properties for methane deep oxidation
Abstract
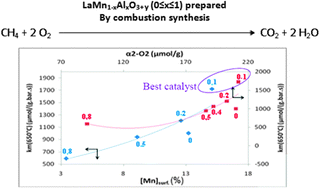
The objective of this work was to study the effect of aluminum incorporation into the B sublattice of lanthanum manganite on the thermal stability and catalytic activity in CH4 complete

 Please wait while we load your content...
Please wait while we load your content...