Tandem inverse-electron-demand hetero-/retro-Diels–Alder reactions for aromatic nitrogen heterocycle synthesis
Abstract
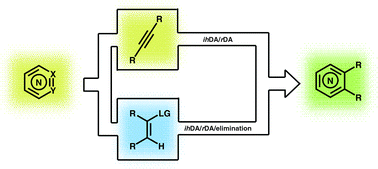
The merged inverse-electron-demand hetero-Diels–Alder (ihDA)/retro-Diels–Alder (rDA) reaction sequence can be used to rapidly synthesise highly functionalised

 Please wait while we load your content...
Please wait while we load your content...