Modeling biofilms with dual extracellular electron transfer mechanisms†
Abstract
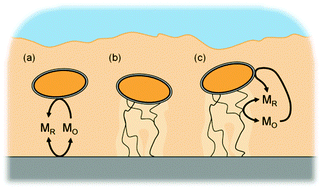
Electrochemically active biofilms have a unique form of respiration in which they utilize solid external materials as terminal electron acceptors for their metabolism. Currently, two primary mechanisms have been identified for long-range extracellular electron transfer (EET): a diffusion- and a conduction-based mechanism. Evidence in the literature suggests that some biofilms, particularly Shewanella oneidensis, produce the requisite components for both mechanisms. In this study, a generic model is presented that incorporates the diffusion- and the conduction-based mechanisms and allows electrochemically active biofilms to utilize both simultaneously. The model was applied to S. oneidensis and Geobacter sulfurreducens biofilms using experimentally generated data found in the literature. Our simulation results show that (1) biofilms having both mechanisms available, especially if they can interact, may have a metabolic advantage over biofilms that can use only a single mechanism; (2) the thickness of G. sulfurreducens biofilms is likely not limited by conductivity; (3) accurate intrabiofilm diffusion coefficient values are critical for current generation predictions; and (4) the local biofilm potential and redox potential are two distinct parameters and cannot be assumed to have identical values. Finally, we determined that simulated cyclic and squarewave voltammetry based on our model are currently not capable of determining the specific percentages of extracellular electron transfer mechanisms in a biofilm. The developed model will be a critical tool for designing experiments to explain EET mechanisms.

 Please wait while we load your content...
Please wait while we load your content...