Heterogeneous and multiphase formation pathways of gypsum in the atmosphere
Abstract
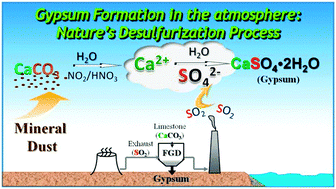
Gypsum is a major sulphur-containing component of atmospheric particulate matter. To date, however, its formation pathways in the atmosphere are still not well known. In this study, several potentially important formation pathways of gypsum in atmospheric aerosols are proposed. We found that gypsum was formed in the humidifying–dehumidifying process of mixed sulphate and calcium salts. A deliquescent layer is crucial for the formation of gypsum from Ca2+ and SO42− ions. In particular, the presence of hygroscopic components, such as (NH4)2SO4 and Ca(NO3)2, is necessary for the conversion of calcium carbonate (CaCO3) upon heterogeneous reaction of either SO2 + O3 or SO2 + NO2 as well as anhydrous calcium sulphate (CaSO4) to form gypsum (CaSO4·2H2O) under ambient conditions. This study provides definitive evidence that synergistic effects in the physical and chemical processing of aerosol particles have a significant effect on their final chemical composition, mixing state and hygroscopic behaviour which dictates the environmental and climate impacts of the resulting aerosol.

 Please wait while we load your content...
Please wait while we load your content...