Room-temperature silver-containing liquid metal salts with nitrate anions†
Abstract
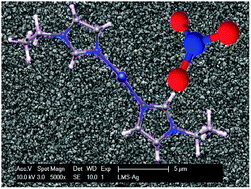
The synthesis, structural, thermal and electrochemical properties of fluorine-free silver-containing ionic liquids are presented. The ionic liquid cations are formed by a silver(I) ion surrounded by two 1-alkylimidazole ligands, with the counter anions being nitrate ions. Depending on the alkyl chain length, the complexes were found to be liquids at room temperature or melting slightly above this. For the solid compounds it was possible to elucidate the structure by single crystal X-ray analysis. The ionic liquids are electroactive, have good mass transport properties and can be used for the electrodeposition of silver at high current densities. The thermal properties and stability of these compounds were tested by differential scanning calorimetry and thermogravimetric analysis. The viscosity of the ionic liquids follows a Vogel–Tamman–Fulcher relationship as a function of temperature. The electrochemical properties of the complexes were tested by cyclic voltammetry and the resulting electrodeposits were examined using scanning electron microscopy and atomic force microscopy.

 Please wait while we load your content...
Please wait while we load your content...