Understanding the evaporation of ionic liquids using the example of 1-ethyl-3-methylimidazolium ethylsulfate
Abstract
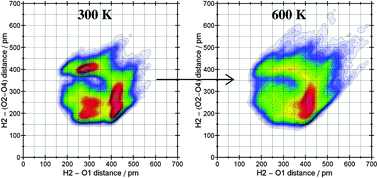
In this work we present a comprehensive temperature-dependence analysis of both the structural and the dynamic properties of a vaporized ionic liquid (1-ethyl-3-methylimidazolium ethylsulfate). This particular ionic liquid is known to be distillable from experimental studies and thus enables us to deepen the understanding of the evaporation mechanism of ionic liquids. We have used ab initio molecular dynamics of one ion pair at three different temperatures to accurately describe the interactions present in this model ionic liquid. By means of radial and spatial distribution functions a large impact on the coordination pattern at 400 K is shown which could explain the transfer of one ion pair from the bulk to the gas phase. Comparison of the free energy surfaces at 300 K and 600 K supports the idea of bulk phase-like and gas phase-like ion pairs. The different coordination patterns caused by the temperature, describing a loosening of the anion side chains, are also well reflected in the power spectra. The lifetime analysis of typical conformations for ionic liquids shows a characteristic behavior at 400 K (temperature close to the experimental evaporation temperature), indicating that conformational changes occur when the ionic liquid is evaporated.
- This article is part of the themed collection: 5th Congress on Ionic Liquids

 Please wait while we load your content...
Please wait while we load your content...