Autocatalytic cathodic dehalogenation triggered by dissociative electron transfer through a C–H⋯O hydrogen bond†
Abstract
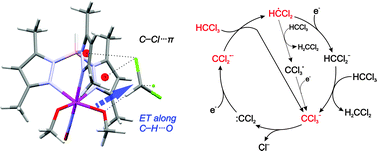
A combined action of the C–H⋯Oalkoxide hydrogen bonding and Cl⋯πpyrazolyl dispersive interactions facilitates intramolecular electron transfer (ET) in the transient {MoI(NO)(TpMe2)(Oalkoxide)}˙−⋯HCCl3 adduct ([TpMe2]− = κ3-hydrotris(3,5-dimethylpyrazol-1-yl)borate), setting off a radical autocatalytic process, eventually leading to chloroform degradation. In the voltammetric curve, this astonishingly fast process is seen as an almost vertical drop-down. The potential at which it occurs is favorably shifted by ca. 1 V in comparison with uncatalyzed reduction. As predicted by DFT calculations, crucial in the initial step is a close and prolonged contact between the electron donor (MoI 4d-based SOMO) and acceptor (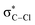 -based LUMO). This occurs owing to the exceptionally short (dH⋯O = 1.82 Å) and nearly linear C–H⋯Oalkoxide bonding, which is reflected by a large ΔνC–H red-shift of 380 cm−1 and a noticeable reorganization of electronic density along the H-bond axis. The advantageous noncovalent interactions inside the cavity formed by two pyrazolyl (pz) rings are strengthened during the C–Cl bond elongation coupled with the ET, giving rise to possible transition state stabilization. After the initial period, the reaction proceeds as a series of consecutive alternating direct or MoII/I-mediated electron and proton transfers. Alcohols inhibit the electrocatalysis by binding with the {MoI–Oalkoxide}˙− active site, and olefins by trapping transient radicals. The proximity and stabilization effects, and competitive inhibition in the studied system may be viewed as analogous to those operating in enzymatic catalysis.
-based LUMO). This occurs owing to the exceptionally short (dH⋯O = 1.82 Å) and nearly linear C–H⋯Oalkoxide bonding, which is reflected by a large ΔνC–H red-shift of 380 cm−1 and a noticeable reorganization of electronic density along the H-bond axis. The advantageous noncovalent interactions inside the cavity formed by two pyrazolyl (pz) rings are strengthened during the C–Cl bond elongation coupled with the ET, giving rise to possible transition state stabilization. After the initial period, the reaction proceeds as a series of consecutive alternating direct or MoII/I-mediated electron and proton transfers. Alcohols inhibit the electrocatalysis by binding with the {MoI–Oalkoxide}˙− active site, and olefins by trapping transient radicals. The proximity and stabilization effects, and competitive inhibition in the studied system may be viewed as analogous to those operating in enzymatic catalysis.

 Please wait while we load your content...
Please wait while we load your content...