Collective hydrogen-bond dynamics dictates the electronic structure of aqueous I3−†
Abstract
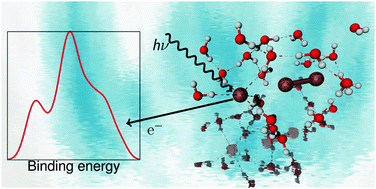
The molecular and electronic structures of aqueous I3− and I− ions have been investigated through ab initio molecular dynamics (MD) simulations and photoelectron (PE) spectroscopy of the iodine 4d core levels. Against the background of the theoretical simulations, data from our I4d PE measurements are shown to contain evidence of coupled solute–solvent dynamics. The MD simulations reveal large amplitude fluctuations in the I–I distances, which couple to the collective rearrangement of the hydrogen bonding network around the I3− ion. Due to the high polarizability of the I3− ion, the asymmetric I–I vibration reaches partially dissociated configurations, for which the electronic structure resembles that of I2 + I−. The charge localization in the I3− ion is found to be moderated by hydrogen-bonding. As seen in the PE spectrum, these soft molecular vibrations are important for the electronic properties of the I3− ion in solution and may play an important role in its electrochemical function.

 Please wait while we load your content...
Please wait while we load your content...