Solvent-mediated folding of dicarboxylate dianions: aliphatic chain length dependence and origin of the IR intensity quenching†
Abstract
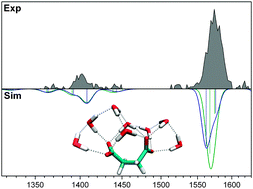
We combine infrared photodissociation spectroscopy with quantum chemical calculations to characterize the hydration behavior of microsolvated dicarboxylate dianions, (CH2)m(COO−)2·(H2O)n, as a function of the aliphatic chain length m. We find evidence for solvent-mediated folding transitions, signaled by the intensity quenching of the symmetric carboxylate stretching modes, for all three species studied (m = 2, 4, 8). The number of water molecules required to induce folding increases monotonically with the chain length and is n = 9–12, n = 13, and n = 18–19 for succinate (m = 2), adipate (m = 4), and sebacate (m = 8), respectively. In the special case of succinate, the structural transition is complicated by the possibility of bridging water molecules that bind to both carboxylates with merely minimal chain deformation. On the basis of vibrational calculations on a set of model systems, we identify the factors responsible for intensity quenching. In particular, we find that the effect of hydrogen bonds on the carboxylate stretching mode intensities is strongly orientation dependent.

 Please wait while we load your content...
Please wait while we load your content...