Hydrogen–fluorine exchange in NaBH4–NaBF4†
Abstract
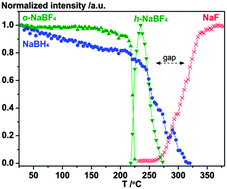
Hydrogen–fluorine exchange in the NaBH4–NaBF4 system is investigated using a range of experimental methods combined with DFT calculations and a possible mechanism for the reactions is proposed. Fluorine substitution is observed using in situ synchrotron radiation powder X-ray diffraction (SR-PXD) as a new Rock salt type compound with idealized composition NaBF2H2 in the temperature range T = 200 to 215 °C. Combined use of solid-state 19F MAS NMR, FT-IR and DFT calculations supports the formation of a BF2H2− complex ion, reproducing the observation of a 19F chemical shift at −144.2 ppm, which is different from that of NaBF4 at −159.2 ppm, along with the new absorption bands observed in the IR spectra. After further heating, the fluorine substituted compound becomes X-ray amorphous and decomposes to NaF at ∼310 °C. This work shows that fluorine-substituted borohydrides tend to decompose to more stable compounds, e.g. NaF and BF3 or amorphous products such as closo-boranes, e.g. Na2B12H12. The NaBH4–NaBF4 composite decomposes at lower temperatures (300 °C) compared to NaBH4 (476 °C), as observed by thermogravimetric analysis. NaBH4–NaBF4 (1 : 0.5) preserves 30% of the hydrogen storage capacity after three hydrogen release and uptake cycles compared to 8% for NaBH4 as measured using Sievert’s method under identical conditions, but more than 50% using prolonged hydrogen absorption time. The reversible hydrogen storage capacity tends to decrease possibly due to the formation of NaF and Na2B12H12. On the other hand, the additive sodium fluoride appears to facilitate hydrogen uptake, prevent foaming, phase segregation and loss of material from the sample container for samples of NaBH4–NaF.

 Please wait while we load your content...
Please wait while we load your content...