A folding transition underlies the emergence of membrane affinity in amyloid-β†
Abstract
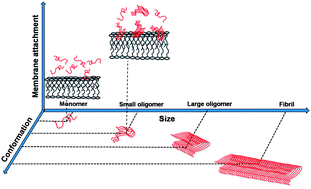
Small amyloid-β (Aβ) oligomers have much higher membrane affinity compared to the monomers, but the structural origin of this functional change is not understood. We show that as monomers assemble into small n-mers (n < 10), Aβ acquires a tertiary fold that is consistent with the mature fibrils. This is an early and defining transition for the aggregating peptide, and possibly underpins its altered bioactivity.

 Please wait while we load your content...
Please wait while we load your content...