Cooperative hydration of carboxylate groups with alkali cations†
Abstract
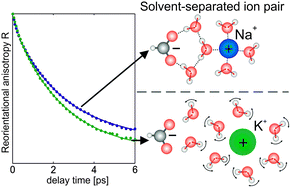
We study the orientational dynamics of water molecules in solutions of formate salts using femtosecond mid-infrared spectroscopy. We observe that combining the formate ion with small cations like Na+ or Li+ leads to a cooperative effect on the water dynamics. This observation points at the formation of solvent-separated ion pairs.

 Please wait while we load your content...
Please wait while we load your content...