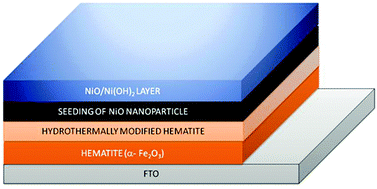
Control of the water splitting reaction in the context of natural photosynthesis is considered as a Holy Grail of chemistry, particularly with respect to artificial photosynthesis for a sustainable energy economy. The underlying objective is to build a solar fuel generator which is economically viable and environmentally benign. Hydrogen generation by solar water splitting in photoelectrochemical cells (PEC) is currently experiencing a renaissance, and the search for high performance but low-cost photoelectrode materials is an on-going quest. We present here a photoanode heterostructure of hematite and NiO/α-Ni(OH)2, which is very efficient. We prepared the heterostructure by a “two reactor” hydrothermal modification of a pristine hematite film. The system shows promising current density of 16 mA cm−2, several times higher than that of the pristine hematite film. In addition, the system shows charge storing capacity once exposed to AM 1.5 simulated sunlight, along with electrochromic behaviour. Interestingly, the water splitting proceeds as a dark reaction after several hours of light exposure. The abrupt increase in current density originates from the oxidized Ni(OH)2 layer which is absent in the case of pn-junction-like devices made by mere deposition of NiO on hematite by thermal annealing. Hematite alone shows no such behaviour. This kind of new PEC electrode offers a low-cost and simple way for the dual purpose applications of water splitting and charge storage.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...