Activation of BODIPY fluorescence by the photoinduced dealkylation of a pyridinium quencher
Abstract
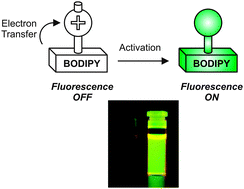
The photoinduced cleavage of a 2-nitrobenzyl group from a pyridinium quencher covalently attached to the meso position of a BODIPY fluorophore activates the emission of the latter. This photochemical transformation prevents the transfer of one electron from the BODIPY platform to its heterocyclic appendage upon excitation and, as a result, permits the radiative deactivation of the excited fluorophore. This versatile mechanism for fluorescence switching can translate into the realization of an entire family of photoactivatable fluorophores based on the outstanding photophysical properties of BODIPY chromophores.
- This article is part of the themed collection: Superresolution imaging and fabrication with light

 Please wait while we load your content...
Please wait while we load your content...