Redox and electrochemical water splitting catalytic properties of hydrated metal oxide modified electrodes†
Abstract
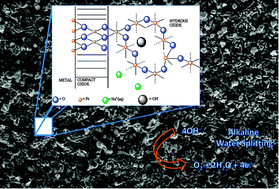
This paper presents a review of the redox and electrocatalytic properties of transition metal oxide electrodes, paying particular attention to the oxygen evolution reaction. Metal oxide materials may be prepared using a variety of methods, resulting in a diverse range of redox and electrocatalytic properties. Here we describe the most common synthetic routes and the important factors relevant to their preparation. The redox and electrocatalytic properties of the resulting oxide layers are ascribed to the presence of extended networks of hydrated surface bound oxymetal complexes termed surfaquo groups. This interpretation presents a possible unifying concept in water oxidation catalysis – bridging the fields of heterogeneous electrocatalysis and homogeneous molecular catalysis.

 Please wait while we load your content...
Please wait while we load your content...