Nuclear magnetic resonance study of ion adsorption on microporous carbide-derived carbon†
Abstract
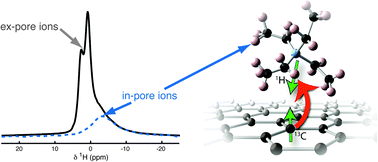
A detailed understanding of ion
* Corresponding authors
a
Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, UK
E-mail:
cpg27@cam.ac.uk
b Department of Chemistry, Stony Brook University, Stony Brook, NY, USA
c INM – Leibniz-Institute for New Materials, Energy Materials Group, Campus D2 2, D-66123 Saarbrücken, Germany
d Department of Materials Science and Engineering and A.J. Drexel Nanotechnology Institute, Drexel University, Philadelphia, USA
e Université Paul Sabatier, CIRIMAT UMR CNRS 5085 Toulouse, 31062, France and Réseau sur le Stockage Electrochimique de l'Energie (RS2E), FR CNRS 3459, France
A detailed understanding of ion

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
A. C. Forse, J. M. Griffin, H. Wang, N. M. Trease, V. Presser, Y. Gogotsi, P. Simon and C. P. Grey, Phys. Chem. Chem. Phys., 2013, 15, 7722 DOI: 10.1039/C3CP51210J
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence. You can use material from this article in other publications without requesting further permissions from the RSC, provided that the correct acknowledgement is given.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
