Theoretical calculations of stability constants and pKa values of metal complexes in solution: application to pyridoxamine–copper(ii) complexes and their biological implications in AGE inhibition†
Abstract
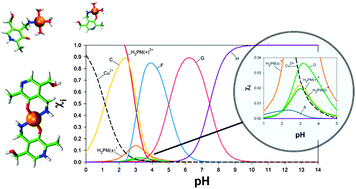
Accurate prediction of thermodynamic constants of chemical reactions in solution is one of the current challenges in computational chemistry. We report a scheme for predicting stability constants (log β) and pKa values of metal complexes in solution by means of calculating free energies of ligand- and proton-exchange reactions using Density Functional Theory calculations in combination with a continuum solvent model. The accuracy of the predicted log β and pKa values (mean absolute deviations of 1.4 and 0.2 units respectively) is equivalent to the experimental uncertainties. This theoretical methodology provides direct knowledge of log β and pKa values of major and minor species, so it is of potential use in combination with experimental techniques to obtain a detailed description of the microscopic equilibria. In particular, the proposed methodology is shown to be especially useful for obtaining the real acidity constants of those chelates where the metal–ligand coordination changes as a result of ligand deprotonation. The stability and acidity constants of pyridoxamine–Cu2+ chelates calculated with the proposed methodology show that pyridoxamine is an efficient scavenging agent of Cu2+ under physiological pH conditions. This is of special interest as Cu2+ overload is involved in the formation of advanced glycation end-products (AGEs) and their associated degenerative medical conditions.

 Please wait while we load your content...
Please wait while we load your content...