Temperature and composition dependence of kinetics of phase separation in solid binary mixtures
Abstract
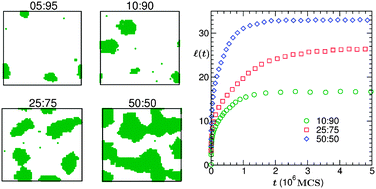
We present results on the effects of temperature variation and composition in the kinetics of phase separation in solid binary mixtures (A1 + A2) from Monte Carlo simulations of the Ising model in two dimensions. The simulation results are understood via an appropriate application of the finite-size scaling theory. At moderately high temperatures, for symmetric (50 : 50) compositions of A1 and A2 particles the average size of the domains exhibits power-law growth with the exponent having a Lifshitz–Slyozov value of 1/3 from very early time. However, our analysis shows that for low enough temperatures, the growth exponent at an early time is smaller than the Lifshitz–Slyozov value. For composition dependence, we find that at moderate temperature, even for extreme off-critical composition, the curvature dependent correction to the growth law is weak which is counter-intuitive in case of droplet morphology. This is, however, consistent with the recent understanding on the curvature dependence of surface tension. Results from rather general studies on the finite-size effects with the variations of temperature and composition have also been presented.

 Please wait while we load your content...
Please wait while we load your content...