Thermal unfolding and refolding of lysozyme in deep eutectic solvents and their aqueous dilutions†
Abstract
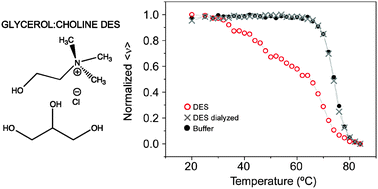
The stability of hen's egg white lysozyme in different choline chloride-based pseudo-concentrated and neat deep eutectic solvents (DESs) has been studied by means of intrinsic fluorescence and CD spectroscopy. Thermal unfolding experiments carried out in non-diluted urea:choline chloride and glycerol:choline chloride eutectic solvents (UCCl-DES and GCCl-DES, respectively) showed the accumulation at certain temperatures of discrete, partially folded intermediates that displayed a high content of secondary structure and a disrupted tertiary structure. Reversibility of the unfolding process was incomplete in these circumstances, with the urea-based DES showing higher protein structure destabilization upon thermal treatment. On the other hand, aqueous dilution of the eutectic mixtures allowed the recovery of a reversible, two-state denaturation process. Lysozyme activity was also affected in neat and pseudo-concentrated GCCl-DES, with an increasing recovery of activity upon aqueous dilution, and full restoration after DES removal through extensive dialysis. These results suggest that protein interactions at room temperature are reversible and depend on the DES components and on the aqueous content of the original DES dilution.

 Please wait while we load your content...
Please wait while we load your content...