Pressure and temperature dependent photolysis of glyoxal in the 355–414 nm region: evidence for dissociation from multiple states†
Abstract
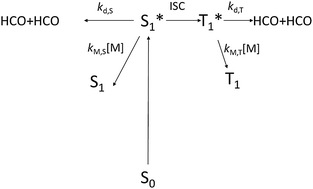
The photolysis of glyoxal has been investigated in the 355–414 nm region by dye laser photolysis coupled with cavity ring-down spectroscopy. Absolute quantum yields of HCO, ΦHCO, were determined using the reaction of chlorine atoms with formaldehyde as an actinometer. The dependence of the quantum yield on pressure was investigated in 3–400 Torr of nitrogen buffer gas and at four temperatures: 233 K, 268 K, 298 K and 323 K. For 355 nm ≤ λ < 395 nm the HCO quantum yield is pressure dependent with linear Stern–Volmer (SV) plots (1/ΦHCOvs. pressure). The zero pressure quantum yield, obtained by extrapolation of the SV plots, rises from 1.6 to 2 between 355 and 382 nm and remains at 2 up to 395 nm. For λ ≥ 395 nm ΦHCO shows a stronger pressure dependence and non-linear SV plots, compatible with formation of HCO by dissociation from two electronic states of glyoxal with significantly different lifetimes. These observations are used to develop a mechanism for the photolysis of glyoxal over the wavelength range studied.

 Please wait while we load your content...
Please wait while we load your content...