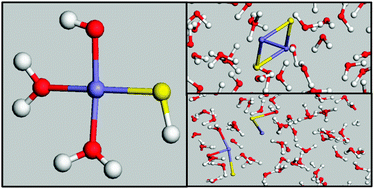
Density Functional Theory-based calculations have been employed to investigate the structure, stability and dynamics of iron sulphide clusters, FexSy (x, y ≤ 4), in water. Car–Parrinello molecular dynamics simulations of the building unit FeS in explicit water show that the iron is only four-coordinated, which indicates that the effect of sulphur is to significantly reduce the coordination shell of iron compared with the typical octahedral arrangement of hexa-aqua iron complexes in water. The molecular dynamics simulations of FexSy particles (x, y ≥ 2) in explicit water reveal that these clusters are highly unstable as they dissociate after a few picoseconds. The Gibbs free energies to form the FeS and Fe2S2 species have been evaluated in a simulated aqueous environment, using the mPW1B95 density functional theory level for the gas-phase component and the UAHF-CPCM solvation model for the hydration contribution, and the results indicate that while FeS is thermodynamically stable in aqueous solution, the formation of a Fe2S2 cluster is endergonic, and dissociation is preferred under natural water conditions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...