An electrochemical impedance study of the oxygen evolution reaction at hydrous iron oxide in base†
Abstract
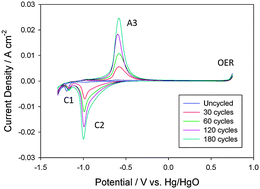
The oxygen evolution reaction at multi-cycled iron oxy-hydroxide films in aqueous alkaline solution is discussed. Steady-state Tafel plot analysis and electrochemical impedance spectroscopy have been used to elucidate the kinetics and mechanism of oxygen evolution. Tafel slopes of ca. 60 mV dec−1 and 40 mV dec−1 are found at low overpotentials depending on the oxide growth conditions, with an apparent Tafel slope of ca. 120 mV dec−1 at high overpotentials. Reaction orders of ca. 0.5 and 1.0 are observed at low and high overpotentials, again depending on the oxide growth conditions. A mechanistic scheme involving the active participation of octahedrally coordinated anionic iron oxyhydroxide surfaquo complexes, which form the porous hydrous layer, is proposed. The latter structure contains considerable quantities of water molecules which facilitate hydroxide ion discharge at the metal site during active oxygen evolution. This work brings together current research in heterogeneous electrocatalysis and homogeneous molecular catalysis for water oxidation.
- This article is part of the themed collection: Electrochem 2012 "Electrochemical Horizons"

 Please wait while we load your content...
Please wait while we load your content...