A theoretical study of the two binding modes between lysozyme and tri-NAG with an explicit solvent model based on the fragment molecular orbital method
Abstract
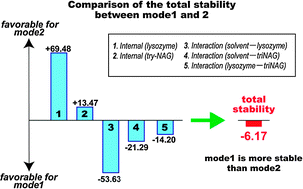
To examine the stabilities and binding characteristics, fragment molecular orbital (FMO) calculations were performed for the two binding modes of hen egg-white lysozyme with tri-N-acetyl-D-glucosamine (tri-NAG).

 Please wait while we load your content...
Please wait while we load your content...