What stabilizes close arginine pairing in proteins?†
Abstract
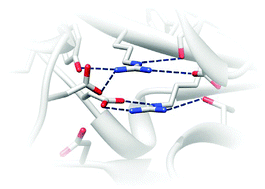
Close stacking of arginine residues are often observed in protein structures despite the highly repulsive nature of the close like-charged groups. Physical factors stabilizing the close guanidinium ions of arginine side-chains have been previously studied in water and in protein-like environments, and the hydration free energy has been emphasized to be an important factor. However, how close arginine pairs are stabilized in real proteins has not been fully understood yet. In this paper, we show that arginine pairs are more frequently found in the protein interior than expected from the frequency of unpaired arginines buried inside protein through a statistical analysis of the protein structure database. We then confirm that 4 selected arginine pairs buried in the protein are indeed positively charged rather than neutralized, by molecular dynamics simulations and pKa estimation with molecular mechanics–Poisson–Boltzmann calculations. Further energy decomposition analysis shows that the hydration free energy may not be strong enough to overcome the repulsive Coulomb interaction between the positively charged arginine residues buried inside the protein. Instead, a highly polar interaction network is identified around each buried arginine pair, and the electrostatic interactions within such network are strong enough to stabilize the repulsive interaction of the buried arginine pair for the 4 selected cases. The polar interaction network is highly conserved evolutionarily in some proteins, implicating their roles in protein stabilization or biochemical function.

 Please wait while we load your content...
Please wait while we load your content...