Evidence for lithium superoxide-like species in the discharge product of a Li–O2 battery†
Abstract
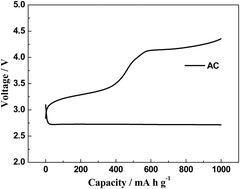
We report on the use of a petroleum coke-based activated carbon (AC) with very high surface area for a Li–O2 battery cathode without the use of any additional metal

 Please wait while we load your content...
Please wait while we load your content...