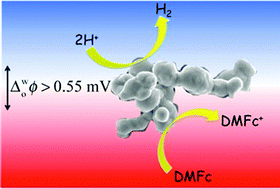
Rarely reported low-cost molybdenum boride and carbide microparticles, both of which are available in abundant quantities due to their widespread use in industry, adsorb at aqueous acid–1,2-dichloroethane interfaces and efficiently catalyse the hydrogen evolution reaction in the presence of the organic electron donor – decamethylferrocene. Kinetic studies monitoring biphasic reactions by UV/vis spectroscopy, and further evidence provided by gas chromatography, highlight (a) their superior rates of catalysis relative to other industrially significant transition metal carbides and silicides, as well as a main group refractory compound, and (b) their highly comparable rates of catalysis to Pt microparticles of similar dimensions. Insight into the catalytic processes occurring for each adsorbed microparticle was obtained by voltammetry at the liquid–liquid interface.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...