Benchmark calculations of metal carbonyl cations: relativistic vs. electron correlation effects
Abstract
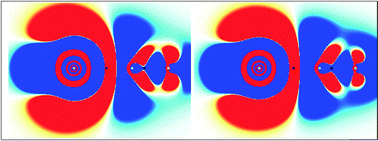
In this paper we present benchmark results for isoelectronic metal carbonyl complexes of the groups 11 and 12 of the periodic table. The focus is on the geometry, vibrational frequencies, bond dissociation energy and chemical bonding. The description of these complexes requires a good balance between electron correlation and relativistic effects. Our results demonstrate that the combination of the effective core potential and the MP2 method gives quantitative results for the first- and the second-row transition metal complexes and only qualitative agreement for the third-row complexes. In order to obtain quantitative results for the whole series the use of four-component or X2C methods is mandatory. The fourth-row transition metal carbonyl complexes from groups 11 and 12 have been studied for the first time. The metal–carbon bond strength pattern along the group is shown to be highly dependent on the correct description of the relativistic effects. Finally, the relativistic effects on the bonding are studied by means of electron density difference maps, the analysis of the bond critical points of the electron density and the mechanism for σ-donation and π-backdonation. Our analysis indicates that the fourth-row complexes exhibit a strong covalent character induced by relativistic effects.

 Please wait while we load your content...
Please wait while we load your content...