Synthesis of Bi6Mo2O15 sub-microwires via a molten salt method and enhancing the photocatalytic reduction of CO2 into solar fuel through tuning the surface oxide vacancies by simple post-heating treatment †
Abstract
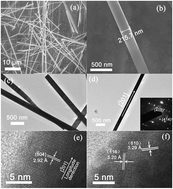
Monoclinic phase Bi6Mo2O15 sub-microwires consisting of MoO4 tetrahedra have been successfully synthesized by a molten salt method. The wide-bandgap sub-microwire exhibits photocatalytic activity toward the photoreduction of CO2 into CH4. The existence of surface oxide vacancies enhanced the photocatalytic activity, which can be easily tuned via different post-heating temperatures, through capturing photo-generated electrons at the surface, thus being beneficial for the separation of electrons and holes and prolonging the lifetime of the electrons.

 Please wait while we load your content...
Please wait while we load your content...