Halogen bonding versus hydrogen bonding: what does the Cambridge Database reveal?†
Abstract
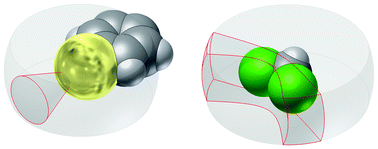
A novel procedure for the thorough analysis of intermolecular interactions from structural data found in the Cambridge Structural Database has been developed, which takes into account the amount of space occupied by the interacting partners. Hence, volume-corrected probability distribution plots and relative abundance plots have been generated for molecule⋯ElR pairs (where molecule is a p-X-phenyl group with X = CH3, F, Cl, Br, I or

 Please wait while we load your content...
Please wait while we load your content...