Crystallographic and ab initio study of pyridine CH–O interactions: linearity of the interactions and influence of pyridine classical hydrogen bonds†
Abstract
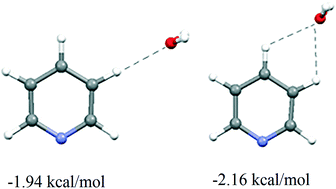
The CH–O interactions of pyridine with water molecules were studied by analysing the data in the Cambridge Structural Database (CSD) and by ab initio calculations. The analysis of the CH–O interactions in the crystal structures from the CSD indicates that pyridine C–H donors do not show preference for linear contacts. The results of the ab initio calculations are in accord with the CSD data and show that stabilization energy is larger for bifurcated interactions than for linear interactions. The calculated interaction energies at the MP2/cc-pVQZ level for linear CH–O interactions between water and pyridine ortho, meta, and para C–H groups are −1.24, −1.94 and −1.97 kcal mol−1, respectively. The calculated energies for bifurcated ortho–meta and meta–para interactions are −1.96 and −2.16 kcal mol−1. The data in the crystal structures from the CSD and ab initio calculations show a strong influence of simultaneous classical hydrogen bonds of pyridine on the CH–O interactions. The results show that simultaneous hydrogen bonds strengthen the CH–O interaction by about 20%. The calculated interaction energies for linear CH–O interactions between water and pyridine, with simultaneous hydrogen bonds, for ortho, meta, and para C–H groups are −1.64, −2.34, and −2.33 kcal mol−1, respectively, while those for ortho–meta and meta–para bifurcated interactions are −2.44 and −2.58 kcal mol−1. The energies of the meta–para bifurcated interactions calculated at the CCSD(T)(limit) level for pyridine without and with hydrogen bonds are −2.30 and −2.69 kcal mol−1, respectively. The result that nonlinear interactions are energetically favoured can be very important for recognizing the CH–O interaction of heteroaromatic rings in the crystal structures and biomolecules.

 Please wait while we load your content...
Please wait while we load your content...