Halogen bonding versuschalcogen and pnicogen bonding: a combined Cambridge structural database and theoretical study†
Abstract
In this manuscript we analyze the Cambridge Structural Database (
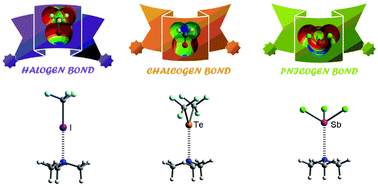
- This article is part of the themed collection: Halogen bonding: from self-assembly to materials and biomolecules

 Please wait while we load your content...
Please wait while we load your content...