Chemoaffinity-mediated crystallization of Cu2O: a reaction effect on crystal growth and anode property†
Abstract
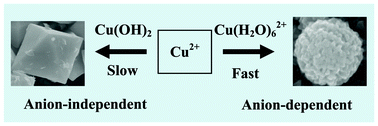
We present a chemoaffinity-mediated crystallization strategy to fabricate Cu2O with different morphologies for studying their electrochemical performances as lithium-ion battery anode materials. The chemical reaction routes were finely-designed by including important anions such as OH−, SO42−, NO3−, Ac−, and Cl− in the redox system of N2H4 and Cu2+. With different affinity abilities of the anions in terms of hard–soft acid–base theory, two growth mechanisms were evidenced, which can lead to the crystallization of spherical aggregations and octahedra, respectively. The crystallization of Cu2O octahedra occurred in the presence of OH−, which is independent of anions, due to the higher chemoaffinity of OH− with Cu2+. Without adding additional OH−, anions such as SO42−, NO3−, Ac−, and Cl− can induce diverse chemical reactions to effectively control the growth of Cu2O spherical aggregations. Electrochemical measurements indicated that Cu2O octahedra can show better cyclability than that of spherical aggregations. The present work validates that the chemoaffinity-mediated method is a useful bottom-up strategy to crystallize high performance anode materials.

 Please wait while we load your content...
Please wait while we load your content...