An efficient synthetic route to carbocyclic enaminonitriles via Lewis acid catalysed domino-ring-opening-cyclisation (DROC) of donor–acceptor cyclopropanes with malononitrile†
Abstract
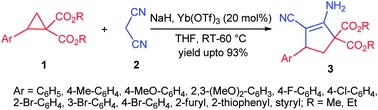
A highly efficient strategy for the synthesis of functionalised carbocyclic enaminonitriles in excellent yields has been described. The reaction utilises Yb(OTf)3 catalysed C–C bond cleavage and two simultaneous C–C bond formations of donor–acceptor (DA)-cyclopropanes with malononitrile anions in a domino fashion.

 Please wait while we load your content...
Please wait while we load your content...