A bifunctional palladium/acid solid catalyst performs the direct synthesis of cyclohexylanilines and dicyclohexylamines from nitrobenzenes†
Abstract
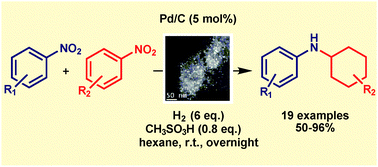
Nitroderivatives are transformed to cyclohexylanilines at room temperature in good yields and selectivity via a hydrogenation-

 Please wait while we load your content...
Please wait while we load your content...