Facile synthesis of dibranched conjugated dienes viapalladium-catalyzed oxidative coupling of N-tosylhydrazones†
Abstract
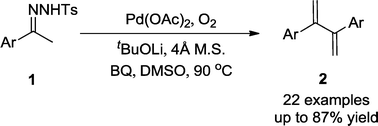
A facile and highly regioselective Pd-catalyzed oxidative coupling of N-tosylhydrazones providing efficient access to 2,3-disubstituted-1,3-butadienes has been developed. This process features readily available starting materials and mild reaction conditions. Further transformations of the obtained dibranched 1,3-dienes, through Diels–Alder reactions and

 Please wait while we load your content...
Please wait while we load your content...